Phagocidal macrophages
Loss of major histocompatibility complex (MHC) class I and interferon (IFN)g sensing are major causes of primary and acquired resistance to checkpoint blockade immunotherapy. Additional treatment options are needed for tumors that lose expression of MHC class I. IAP antagonism can be used to skew cytokine production by T cells and has pleiotropic effects across other immune cell types by mimicking signaling through TNF superfamily receptors. We tested the effect of IAP antagonists in multiple models of highly refractory pancreatic cancer. Induction of non-canonical NF-kB signaling induces T cell-dependent immune responses, even in beta-2-microglobulin (b2M)-deficient tumors, demonstrating that direct CD8 T cell recognition of tumor cell expressed MHC class I is not required. Instead, T cell-produced lymphotoxin reprograms both mouse and human macrophages to be tumoricidal. In wild type mice, but not mice incapable of antigen-specific T cell responses, cIAP1/2 antagonism reduces tumor burden by increasing phagocytosis of live tumor cells. Knowing the mechanism of action inspired combination therapy with CD47 blockade, which resulted in beneficial responses in multiple models of pancreatic cancer where checkpoint blockade fails. Thus, activation of non-canonical NF-kB stimulates a T cell-macrophage axis that curtails growth of tumors that are resistant to checkpoint blockade due to loss of MHC class I or IFNg sensing.
Loss of major histocompatibility complex (MHC) class I and interferon (IFN)g sensing are major causes of primary and acquired resistance to checkpoint blockade immunotherapy. Additional treatment options are needed for tumors that lose expression of MHC class I. IAP antagonism can be used to skew cytokine production by T cells and has pleiotropic effects across other immune cell types by mimicking signaling through TNF superfamily receptors. We tested the effect of IAP antagonists in multiple models of highly refractory pancreatic cancer. Induction of non-canonical NF-kB signaling induces T cell-dependent immune responses, even in beta-2-microglobulin (b2M)-deficient tumors, demonstrating that direct CD8 T cell recognition of tumor cell expressed MHC class I is not required. Instead, T cell-produced lymphotoxin reprograms both mouse and human macrophages to be tumoricidal. In wild type mice, but not mice incapable of antigen-specific T cell responses, cIAP1/2 antagonism reduces tumor burden by increasing phagocytosis of live tumor cells. Knowing the mechanism of action inspired combination therapy with CD47 blockade, which resulted in beneficial responses in multiple models of pancreatic cancer where checkpoint blockade fails. Thus, activation of non-canonical NF-kB stimulates a T cell-macrophage axis that curtails growth of tumors that are resistant to checkpoint blockade due to loss of MHC class I or IFNg sensing.
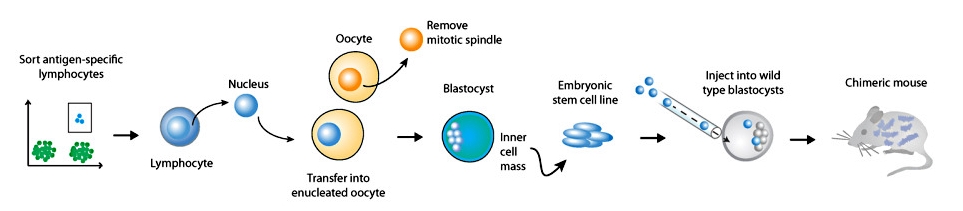
Transnuclear mice
Somatic cell nuclear transfer (SCNT) may be used to clone mice from lymphocytes of defined specificity. By harvesting as few as 200 primary lymphocytes from animals that are at the peak of an immune response, and by transfer of the nucleus from such antigen specific lymphocytes into an enucleated oocyte, embryonic stem cells that harbor the genetic rearrangements encoding the original antigen receptor may be obtained and used for the construction of transnuclear mice. These animals contain T or B cells of the appropriate specificity, have no genetic alterations other than the physiological TCR/BCR rearrangements and are the closest approximation of physiological immune responses to date. Importantly, the generation of transnuclear mice is rapid, requiring approximately 6 weeks from T cell harvest to obtaining chimeric animals.
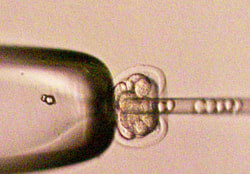 Dougan lab injecting embryonic stem cells into mouse embryos
Dougan lab injecting embryonic stem cells into mouse embryos
CRISPR genome-edited mice
We use the Cas9-CRISPR system to directly modify genes in fertilized mouse zygotes. We have optimized the process to increase the frequency of homologous recombination, thereby generating precise genome edits and decreasing off-target effects.
We use the Cas9-CRISPR system to directly modify genes in fertilized mouse zygotes. We have optimized the process to increase the frequency of homologous recombination, thereby generating precise genome edits and decreasing off-target effects.
Immunotherapy for pancreatic cancer
Pancreatic cancer is one of the deadliest cancers, with a 5 year survival rate of 7%. The exceptionally poor prognosis of this disease can be traced to several factors. Located in a vital region of the body, the majority of primary pancreatic tumors are inoperable due to invasion of the surrounding vasculature. The disease is rapidly metastatic, with most patients presenting with metastases upon initial diagnosis. Finally, pancreatic cancers are dense, fibrotic masses that preclude adequate drug delivery.
Immunotherapy has shown impressive clinical benefit, particularly for metastatic melanoma. The FDA has approved three immune-modulating drugs that target activation inhibitors on T cells, ipilimumab (anti-CTLA-4) and nivolumab/pembrolizumab (anti-PD-1). Since immunotherapy targets the immune system, and not a particular type of cancer, these new drugs were originally hoped to be applicable across all tumor types as a pan-cancer medication. Indeed, significant clinical results have been seen for ipilimumab and PD-1 blockade in lung, renal, prostate, and multiple cancer types deficient in mismatch repair. However, pancreatic cancer was among the cancer types in which ipilimumab and nivolumab have largely failed.
Why has pancreatic cancer been so refractory to immunotherapy? In part, we do not fully understand how tumor-specific T cells infiltrate densely fibrotic pancreatic tumors, or how to recruit more activated T cells to the tumor mass. The Dougan lab is developing novel strategies to address this question.
Immunotherapy has shown impressive clinical benefit, particularly for metastatic melanoma. The FDA has approved three immune-modulating drugs that target activation inhibitors on T cells, ipilimumab (anti-CTLA-4) and nivolumab/pembrolizumab (anti-PD-1). Since immunotherapy targets the immune system, and not a particular type of cancer, these new drugs were originally hoped to be applicable across all tumor types as a pan-cancer medication. Indeed, significant clinical results have been seen for ipilimumab and PD-1 blockade in lung, renal, prostate, and multiple cancer types deficient in mismatch repair. However, pancreatic cancer was among the cancer types in which ipilimumab and nivolumab have largely failed.
Why has pancreatic cancer been so refractory to immunotherapy? In part, we do not fully understand how tumor-specific T cells infiltrate densely fibrotic pancreatic tumors, or how to recruit more activated T cells to the tumor mass. The Dougan lab is developing novel strategies to address this question.